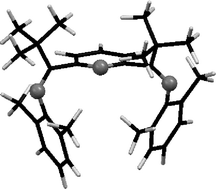
A new bis(imino)pyridine compound, 2,6-{(2,6-Me2-C6H3)NC(t-Bu)}2C5H3N (6), has been synthesized with t-butyl substituents on the imino carbon atoms. The stepwise synthetic method for assembly of this compound is novel. Compound 6 and its synthetic precursor, mono(imino)pyridine 3, have been characterized using single-crystal X-ray diffraction. Metalation attempts of 6 using iron(II) chloride under forcing conditions does not yield the desired iron(II) chloride complex; the use of refluxing acetic acid solvent provides a minimal amount of a paramagnetic species that has been characterized by NMR spectroscopy and magnetic susceptibility (NMR method). Computational methods have been used to evaluate the relative energies of three conformations of bis(imino)pyridine ligands with varying alkyl substitution at the imino carbon positions. The relative energies of these closed, open and open-planar conformations of 6 reveal a thermodynamic argument for the difficulty in metalation of 6, as compared to related ligands with less steric hindrance at the imino carbon atoms.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...