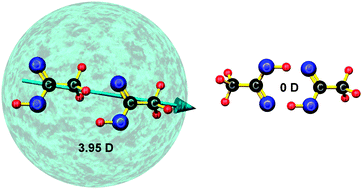
The effect of incident electrons on acetic acid clusters is explored for the first time. The acetic acid clusters are formed inside liquid helium nanodroplets and both cationic and anionic products ejected into the gas phase are detected by mass spectrometry. The cation chemistry (induced by electron ionization at 100 eV) is dominated by production of protonated acetic acid (Ac) clusters, AcnH+, although some fragmentation is also observed. In the case of anion production (at 2.8 eV electron energy) there is a clear distinction between the monomer and the clusters. For the monomer the dominant product is the dehydrogenated species, [Ac–H]−, whereas for the clusters both the parent anion, Acn−, and the dehydrogenated species, [Acn–H]−, have similar abundances. A particularly intriguing contrast between the monomer and cluster anions is that helium atoms are seen attached to the latter whereas no evidence of helium atom attachment is found for the monomer. This surprising observation is attributed to the formation of acyclic (head-to-tail) acetic acid clusters in helium nanodroplets, which have more favourable electronic properties for binding helium atoms. The acyclic clusters represent a local minimum on the potential energy surface and in the case of the dimer this is distinct from the cyclic isomer (the global minimum) identified in gas phase experiments.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...