Diffusion coefficients and local structure in basic molten fluorides: in situNMR measurements and molecular dynamics simulations†
Abstract
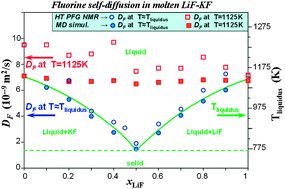
The local structure and the dynamics of molten LiF–KF mixtures have been studied by nuclear magnetic resonance (

 Please wait while we load your content...
Please wait while we load your content...