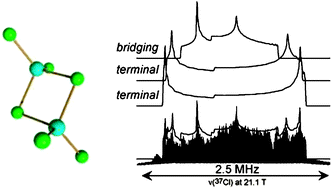
A series of four anhydrous group 13 chloride salts has been studied by 35/37Cl solid-state NMR spectroscopy and complementary quantum chemical calculations. Due to the large 35/37Cl quadrupolar interactions in these salts, a high magnetic field (21.1 T) and the variable-offset QCPMG technique was used to obtain full chlorine central transition (m = −1/2 ↔ 1/2) NMR spectra. Analyses of the NMR spectra of the synthetically important Lewis acid trichlorides of aluminium, gallium, and indium, as well as gallium dichloride, allowed for characterisation of the chlorine electric field gradient (EFG) tensors and, in three cases, the chlorine chemical shift (CS) tensors. The quadrupolar interaction was found to dominate the central transition chlorine NMR spectrum in all cases, with chlorine-35 quadrupolar coupling constants (CQ) ranging in magnitude from 22.45 ± 2.00 to 40.44 ± 2.00 MHz, and the spectral breadths ranging from approximately 1.0 to 2.5 MHz. For the trichloride salt of gallium, it was confirmed that the terminal chlorine sites exhibit larger chlorine CQ values than do the bridging chlorines. The isotropic chemical shifts range from 150 ± 100 to 375 ± 100 ppm while the largest CS tensor span is 500 ± 200 ppm, for InCl3. The chlorine chemical shift was found to increase with increasing M–Cl distance in all cases. Quantum chemical calculations of the EFG and magnetic shielding tensors, performed using the gauge-including projector-augmented-wave (GIPAW) method as implemented in the CASTEP program, were found to be in excellent agreement with the experimentally determined values, reproducing CQ(35Cl) to within 7% in all cases. The agreement between experiment and theory substantiates the accuracy of the NMR parameters. Solid-state NMR spectra of the cation species (aluminium-27, gallium-69/71 and indium-113/115) were also collected, and the EFG and CS parameters were determined in some cases. The study demonstrates the utility of multinuclear solid-state magnetic resonance studies of half-integer spin quadrupolar nuclei in ionic systems when the central transition is broadened greatly by the quadrupolar interaction, and in particular contributes to our understanding of the relationship between solid-state structure and chlorine NMR interaction tensors.

 Please wait while we load your content...
Please wait while we load your content...