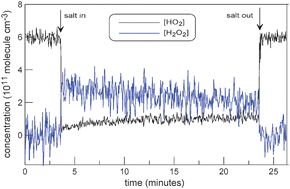
The uptake of HO2 radicals on synthetic sea salt, NaCl, NaBr and MgCl2·6H2O dry solid films was studied over the temperature range 240 to 340 K and at 1 Torr pressure of helium using a discharge flow reactor coupled to a modulated molecular beam mass spectrometer. The Arrhenius expressions (calculated with geometric surface area) were obtained for the uptake coefficient of HO2: 3.1 × 10−9 exp[(3990 ± 200)/T], 2.2 × 10−8 exp[(3340 ± 180)/T], 1.2 × 10−8 exp[(3570 ± 180)/T] and 3.8 × 10−5 exp[(1710 ± 60)/T] on sea salt, NaCl, NaBr and MgCl2·6H2O, respectively (quoted uncertainty is 2σ statistical). H2O2 was observed as a main product of HO2 interaction with salt surface indicating a heterogeneous HO2 self reaction mechanism. The results show that the HO2 loss through heterogeneous interaction with salt surface is not sufficiently rapid to explain the observed differences between modeled and measured HO2 concentrations in remote coastal areas.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...