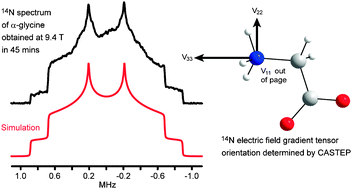
The recently reported direct enhancement of integer spin magnetization (DEISM) methodology for signal enhancement in solid-state NMR of integer spins has been used to obtain static 14N powder patterns from α-glycine, L-leucine and L-proline in relatively short experimental times at 9.4 T, allowing accurate determination of the quadrupolar parameters. Proton decoupling and deuteration of the nitrogen sites were used to reduce the 1H–14N dipolar contribution to the transverse relaxation time allowing more echoes to be acquired per scan. In addition, ab initio calculations using molecular clusters (Gaussian 03) and the full crystal lattice (CASTEP) have been employed to confirm these results, to obtain the orientation of the electric field gradient (EFG) tensors in the molecular frame, and also to correctly assign the two sets of parameters for L-leucine. The 14N EFG tensor is shown to be highly sensitive to the surrounding environment, particularly to nearby hydrogen bonding.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...