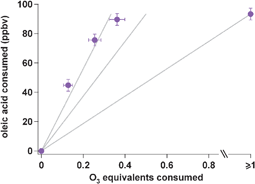
The heterogeneous reaction of ozone with oleic acid has been studied extensively as a simple model system for investigating the oxidation of organic compounds in atmospheric particles. In this work, we simultaneously quantify oleic acid and ozone decay during three stepwise oxidation events, allowing us to quantify reactivity of oleic acid throughout the oxidative lifetime of initially pure particles. Throughout their lifetime, uptake in these particles is driven by reaction, as evidenced by similar timescales for ozone and oleic acid decay. The oleic acid decay rate slows with increasing particle oxidation, most likely due to the continued dilution of the particles with oxidation products. However, the initial stoichiometry is as high as 3.75 oleic acid molecules destroyed per ozone molecule lost. This significantly exceeds the 2 : 1 ratio that can be explained by an initial ozonolysis reaction and known secondary chemistry between the Criegee intermediate and the organic acid moiety. It implies that there is additional, previously unrecognized secondary chemistry that likely involves the carbon backbone. Our understanding of reactivity, even in this simple system, remains incomplete.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...