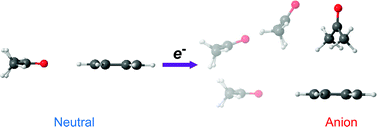
We carried out a comparative study on the anion clusters of naphthalene with various solvents to understand the nature of intermolecular interactions involving an aromatic anion. Photoelectron spectra of mass-selected naphthalene anion clusters, (Np)1−(Sol)n (Sol = benzene, naphthalene, water, and acetone), were obtained, from which the electron affinities were estimated. The electron affinities were significantly different from the vertical detachment energies due to the geometry difference between the neutral and anion clusters along intermolecular coordinates. Theoretical calculations showed that the most stable structure of the naphthalene–acetone anion cluster tends to be a T-shaped geometry because the intermolecular interaction is dominated by π–hydrogen bonding. With the attachment of a second solvent, solvent–solvent interaction was found to compete with ion–solvent interaction for the stable geometries of the anion clusters.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...