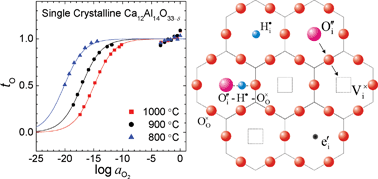
A crystallographic cage structure endows mayenite (Ca12Al14O33 or 12CaO·7Al2O3; C12A7) with remarkable properties, making it either an oxygen solid electrolyte or an inorganic electride upon reduction. In order to better understand the transport properties of C12A7, we measured the equilibrium total conductivity as well as the electronic partial conductivity of single crystal mayenite as functions of activity of oxygen or water vapor at different temperatures in the range 1073 ≤T/K ≤ 1273. A point defect model based on the assumption that the framework [Ca12Al14O32]2+ acts as a pseudo-donor describes well the isothermal conductivity vs.oxygen activity, enabling us to deconvolute the ionic and electronic partial conductivities. The ionic transference number evaluated therefrom clearly demonstrates how C12A7 is converted from a solid electrolyte to an electride depending on the oxygen content. In addition, besides the well known degradation of ionic conductivity by water uptake, a short-term increase of conductivity upon abrupt hydration was recognized and interpreted as due to the transient increase in the concentration of oxygen interstitial along with proton in the initial stage of hydration. For the fully hydrated C12A7, the conductivity relaxation curves upon switching of oxygen activity in a fixed water vapor pressure appear non-monotonic showing the extrema only in the plateau conductivity regime. A defect structure based hypothesis is proposed to explain the 2-fold re-equilibration kinetics.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...