Why can water cages adsorb aqueous methane? A potential of mean force calculation on hydrate nucleation mechanisms
Abstract
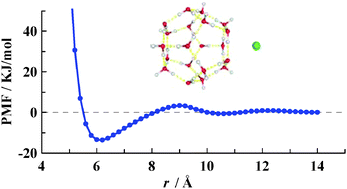
By performing constrained molecular dynamics simulations in the methane–water system, we successfully calculated the potential of mean force (PMF) between a dodecahedral water cage (DWC) and dissolved methane for the first time. As a function of the distance between DWC and methane, this is characterized by a deep well at ∼6.2 Å and a shallow well at ∼10.2 Å, separated by a potential barrier at ∼8.8 Å. We investigated how the guest molecule, cage rigidity and the cage orientation affected the PMF. The most important finding is that the DWC itself strongly adsorbs methane and the adsorption interaction is independent of the guests. Moreover, the activation energy of the DWC adsorbing methane is comparable to that of hydrogen bonds, despite differing by a factor of ∼10% when considering different

 Please wait while we load your content...
Please wait while we load your content...