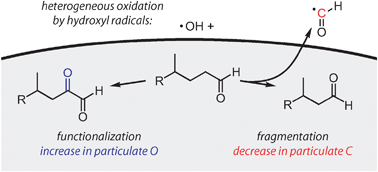
The competition between the addition of polar, oxygen-containing functional groups (functionalization) and the cleavage of C–C bonds (fragmentation) has a governing influence on the change in volatility of organic species upon atmospheric oxidation, and hence on the loading of tropospheric organic aerosol. However the relative importance of these two channels is generally poorly constrained for oxidized organics. Here we determine fragmentation–functionalization branching ratios for organics spanning a range of oxidation levels, using the heterogeneous oxidation of squalane (C30H62) as a model system. Squalane particles are exposed to high concentrations of OH in a flow reactor, and measurements of particle mass and elemental ratios enable the determination of absolute elemental composition (number of oxygen, carbon, and hydrogen atoms) of the oxidized particles. At low OH exposure, the oxygen content of the organics increases, indicating that functionalization dominates, whereas for more oxidized organics the amount of carbon in the particles decreases, indicating the increasing importance of fragmentation processes. Once the organics are moderately oxidized (O/C ≈ 0.4), fragmentation completely dominates, and the increase in O/C ratio upon further oxidation is due to the loss of carbon rather than the addition of oxygen. These results suggest that fragmentation reactions may be key steps in the formation and evolution of oxygenated organic aerosol (OOA).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...