Theoretical investigation of dinitrosyl complexes in Cu-zeolites as intermediates in deNOx process
Abstract
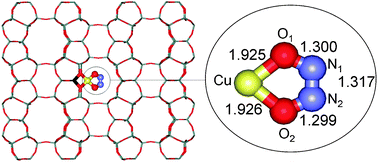
The structure and stability of nitrosyl complexes formed in Cu-FER
- This article is part of the themed collection: In memory of Petr Nachtigall

 Please wait while we load your content...
Please wait while we load your content...