Bifurcation of self-motion depending on the reaction order
Abstract
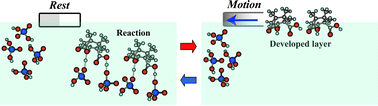
As a simple example of an autonomous motor, the characteristic features of self-motion coupled with the acid–base reaction were numerically and experimentally investigated at the air/aqueous interface. Oscillatory and uniform motion were categorized as a function of the reaction order by numerical computations using a mathematical model that incorporates both the distribution of the surface active layer developed from a material particle as the driving force and the kinetics of the acid–base reaction. The nature of the self-motion was experimentally observed for a boat adhered to a

 Please wait while we load your content...
Please wait while we load your content...