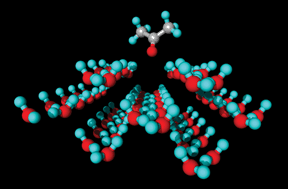
The interaction energies of free acetone molecules with surfaces of two different ice polymorphs have been investigated by quantum chemical methods. Special emphasis has been given to sites for adsorption on the (0001) surface of hexagonal ice (Ih) and the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) surface of cubic ice (Ic), respectively. The structural optimisations made use of conventional electronic structure methods including HF and B3LYP using moderate basis sets up to 6-31+G(d) as well as local and ONIOM methods using 2 or 3 layers which were treated at different levels of theory. The adsorption energies at T = 0 K were calculated for the optimised adsorption geometries performing single points at the B3LYP, MP2 and LMP2 level in conjunction with valence triple-ζ basis sets up to 6-311+G(d,p). Including corrections for basis set superposition errors (BSSE) the most extensive calculations provide adsorption energies (T = 0 K) of −39.1 and −57.5 kJ mol−1 for the energetically most favourable sites for adsorption of a single acetone molecule on ice Ih and ice Ic, respectively. By vibrational analysis this can be transformed to adsorption enthalpies at around a temperature of 200 K yielding values of −31.5 for adsorption on ice Ih and −49.9 kJ mol−1 for adsorption on ice Ic. The current results support experimental observations of Behr et al. (J. Phys. Chem. A, 2006, 110, 8098) in which evidence was presented that acetone adsorbs on ice around 200 K at two different sites; each of which has a different adsorption enthalpy.
01) surface of cubic ice (Ic), respectively. The structural optimisations made use of conventional electronic structure methods including HF and B3LYP using moderate basis sets up to 6-31+G(d) as well as local and ONIOM methods using 2 or 3 layers which were treated at different levels of theory. The adsorption energies at T = 0 K were calculated for the optimised adsorption geometries performing single points at the B3LYP, MP2 and LMP2 level in conjunction with valence triple-ζ basis sets up to 6-311+G(d,p). Including corrections for basis set superposition errors (BSSE) the most extensive calculations provide adsorption energies (T = 0 K) of −39.1 and −57.5 kJ mol−1 for the energetically most favourable sites for adsorption of a single acetone molecule on ice Ih and ice Ic, respectively. By vibrational analysis this can be transformed to adsorption enthalpies at around a temperature of 200 K yielding values of −31.5 for adsorption on ice Ih and −49.9 kJ mol−1 for adsorption on ice Ic. The current results support experimental observations of Behr et al. (J. Phys. Chem. A, 2006, 110, 8098) in which evidence was presented that acetone adsorbs on ice around 200 K at two different sites; each of which has a different adsorption enthalpy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) surface of cubic ice (Ic), respectively. The structural optimisations made use of conventional electronic structure methods including HF and B3LYP using moderate basis sets up to 6-31+G(d) as well as local and ONIOM methods using 2 or 3 layers which were treated at different levels of theory. The
01) surface of cubic ice (Ic), respectively. The structural optimisations made use of conventional electronic structure methods including HF and B3LYP using moderate basis sets up to 6-31+G(d) as well as local and ONIOM methods using 2 or 3 layers which were treated at different levels of theory. The 

 Please wait while we load your content...
Please wait while we load your content...