Stepwise walden inversion in nucleophilic substitution at phosphorus†
Abstract
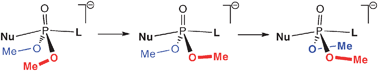
We have studied the mechanism of SN2@P reactions in the model systems X− + PMe2Y and X− + POR2Y (with R = Me, OH, OMe; and X, Y = Cl, OH, MeO) using density functional theory at OLYP/TZ2P. Our main purpose is to analyze the nature of the Walden inversion in our model nucleophilic substitution reactions. Walden inversion is well-known to proceed, in general, as a concerted umbrella motion of the substituents at the central atom. Interestingly, we find here that, in certain model reactions, Walden inversion can also proceed in a stepwise fashion in which the individual substituents of the umbrella flip, consecutively, from the educt to the product conformation via separate barriers on the reaction profile. We also examine how variation in nucleophile and leaving group may tune the pentavalent transition structure between labile transition state (TS) and stable transition complex (TC). Furthermore, we explore the various competing multistep pathways in the symmetric (X = Y) and asymmetric (X ≠ Y) substitution reactions in our model reaction systems.

 Please wait while we load your content...
Please wait while we load your content...