Kinetic studies of atmospherically relevant silicon chemistry
Part I: Silicon atom reactions†
Abstract
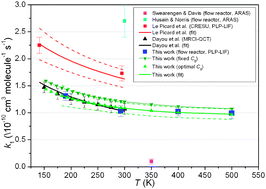
Atomic silicon is generated by meteoric ablation in the Earth’s upper atmosphere (70–110 km). The reactions of Si(3PJ) atoms with several atmospherically relevant species were studied by the pulsed laser photolysis of a Si atom precursor (typically PheSiH3), followed by time-resolved laser induced fluorescence at 251.43 nm (Si(3p23P0→ 4s 3P1)). This yielded: k(Si + O2, 190–500 K) = 9.49 × 10−11 + 1.80 × 10−10× exp(−T/115 K) cm3 molecule−1 s−1 (uncertainty ≤±15%), in good accord with recent high-level theoretical calculations but in marked disagreement with previous experimental work; k(Si + O3, 190–293 K) = (4.0 ± 0.5) × 10−10 cm3 molecule−1 s−1; k(Si + CO2, 293 K) ≤ 1.2 × 10−14 cm3 molecule−1 s−1; and k(Si +

 Please wait while we load your content...
Please wait while we load your content...