Conformations, structural transitions and visible near-infrared absorption spectra of four-, five- and six-coordinated Cu(ii) aqua complexes†
Abstract
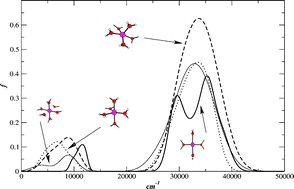
We have performed Car–Parrinello molecular dynamics simulations at ambient conditions for four-, five- and six-coordinated Cu(II) aqua complexes. The molecular geometry has been investigated in terms of Cu–O, Cu–H bond lengths and O–Cu–O bond angles and compared with earlier experimental measurement results and theoretical calculations. We find that the average Cu–O and Cu–H bond lengths increase with increasing coordination number. We have also observed relatively faster structural transition in the case of five-coordinated complex between trigonal bipyramidal and square pyramidal geometry. This result deviates from the findings of the earlier report (A. Pasquarello et al., Science, 2001, 291, 856) on copper(II) in aqueous solution and we attribute these differences to the neglect of

 Please wait while we load your content...
Please wait while we load your content...