Experimental and theoretical evaluation of magnetic coupling in organometallic radicals: the eloquent case of face-to-face Cp⋯Cp interactions
Abstract
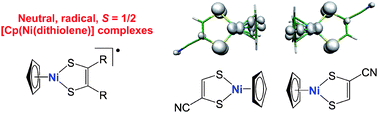
The solid state magnetic properties of an extensive series of neutral radical (S = ½) complexes associating

 Please wait while we load your content...
Please wait while we load your content...