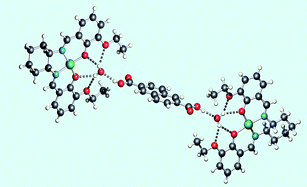
The solid state structures of the copper(II) Schiff base complexes [{Cu(1)}(H2O)], [Cu(1)(OH2)]·MeCN and [({Cu(1)}(H2O))2(2)] (H21 = N,N′-(1R,2R)-(–)-1,2-cyclohexylenebis(3-ethoxysalicylideneamine) and 2 = naphthalene-2,6-dicarboxylic acid) exhibit two recurring structural motifs: host–guest, hydrogen-bonded recognition of H2O in the O,O′,O″,O‴-cavity of [Cu(1)], and face-to-face stacking of [Cu(1)] units with non-bonded Cu⋯Cu separations of 3.54–4.89 Å. In [{Cu(1)}(H2O)], the H2O molecule is non-coordinated to the copper(II) centre whereas in [Cu(1)(OH2)]·MeCN it is axially bound to the copper(II) atom while retaining its role as a hydrogen-bonded guest in the O,O′,O″,O‴-cavity of an adjacent [Cu(1)] molecule. The guest H2O molecule in [{Cu(1)}(H2O)] can function as a hydrogen bond donor as illustrated by the assembly of [({Cu(1)}(H2O))2(2)]. Solution (in THF) electronic spectroscopic data for the complexes are consistent with dissociation of the building blocks including the cleavage of the Cu–OH2 coordination bond. The self-assembly and structural diversity are discussed in terms of a series of hierarchical events.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...