Mechanism of enantioselection in Rh-catalyzed asymmetric hydrogenation. The origin of utmost catalytic performance
Abstract
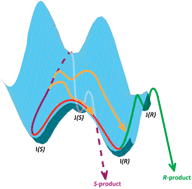
This article describes recent developments in the understanding of the mechanism of enantioselection in one of the most efficient artificial

 Please wait while we load your content...
Please wait while we load your content...