Inhibition of the histone lysine demethylase JMJD2A by ejection of structural Zn(ii)†
Abstract
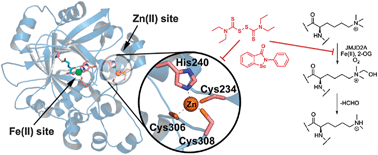
JMJD2A, a 2-oxoglutarate dependent Nε-methyl lysine histone demethylase, is inhibited by disruption of its Zn-binding site by Zn-ejecting compounds including disulfiram and ebselen; this observation may enable the development of inhibitors selective for this subfamily of 2OG dependent oxygenases that do not rely on binding to the highly-conserved Fe(II)-containing active site.

 Please wait while we load your content...
Please wait while we load your content...