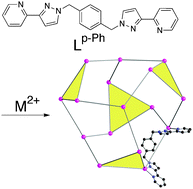
Reaction of simple bis-bidentate ligands, containing two chelating pyrazolyl-pyridine units connected to a central aromatic spacer, with six-coordinate transition metal dications results in self-assembly of an extensive series of polyhedral cage complexes. These include M4L6 tetrahedra, M8L12 cubes, M12L18 truncated tetrahedra and M16L24 tetra-capped truncated tetrahedra. In all cases the metal : ligand ratio is 2 : 3, reflecting the combination of six-coordinate metal ions with tetradentate ligands. The resulting structures are based on those polyhedra which have a 2 : 3 ratio of vertices to faces, with a metal ion at each vertex and bridging ligand spanning each edge. The cages display a range of interesting properties such as an anion-based template effect in the smaller examples; host–guest chemistry associated with the central cavity; aromatic stacking around the periphery between electron-poor and electron-rich ligand fragments which appears to contribute substantially to their stability; and modified fluorescence properties arising from the aromatic stacking of fluorophores such as naphthyl and anthracenyl groups built into the ligand backbone. Even more complex structural types are available using a mixture of face-capping (tris-bidentate) and edge-bridging (bis-bidentate) ligands, such as examples of M12 cuboctahedra which select a combination of two types of ligand during the self-assembly process.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...