Desolvation and substituent effects in edge-to-face aromatic interactions†
Abstract
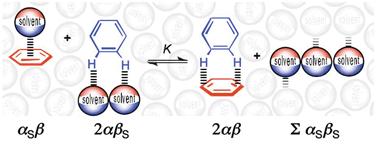
Experimental measurements of aromatic edge-to-face interaction energies in both molecular torsion balances and supramolecular zipper complexes can be reliably estimated using a simple electrostatic solvation model and α/βH-bond constants.

 Please wait while we load your content...
Please wait while we load your content...