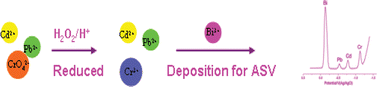
This work herein reports the approach for the simultaneous determination of heavy metal ions including cadmium (Cd(II)), lead (Pb(II)), and chromium (Cr(VI)) using a bismuth film electrode (BFE) by anodic stripping voltammertry (ASV). The BFE used was plated in situ. Due to the reduction of Cr(VI) with H2O2 in the acid medium, on one hand, the Cr(III) was produced and Cr(VI) was indirectly detected by monitoring the content of Cr(III) using square-wave ASV. On the other hand, Pb(II) was also released from the complex between Pb(II) and Cr(VI). Furthermore, the coexistence of the Cd(II) was also simultaneously detected with Pb(II) and Cr(VI) in this system as a result of the formation of an alloy with Bi. The detection limits of this method were 1.39 ppb for Cd(II), 2.47 ppb for Pb(II) and 5.27 ppb for Cr(VI) with a preconcentration time of 120 s under optimal conditions (S/N = 3), respectively. Furthermore, the sensitivity of this method can be improved by controlling the deposition time or by using a cation-exchange polymer (such as Nafion) modified electrode.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...