Regiochemistry of the microwave-assisted reaction between aromatic amines and α-bromoketones to yield substituted 1H-indoles†
Abstract
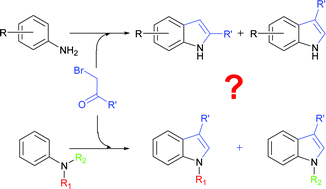
The scope and regioselectivity of the Bischler (or Bischler–Möhlau) reaction between
* Corresponding authors
a
Kimika Fakultatea, Kimika Organika I Saila, Universidad del País Vasco – Euskal Herriko Unibertsitatea, Manuel de Lardizabal Etorbidea 3, San Sebastián-Donostia, Spain
E-mail:
fp.cossio@ehu.es
Fax: +34 943 015270
Tel: +34 943 015442
b Ikerchem Ltd, Tolosa Etorbidea 72, San Sebastián-Donostia, Spain
c Zientzia eta Teknologia Fakultatea, Mineralogia eta Petrologia Saila, Universidad del País Vasco – Euskal Herriko Unibertsitatea, Bilbao, Spain
The scope and regioselectivity of the Bischler (or Bischler–Möhlau) reaction between

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
Y. Vara, E. Aldaba, A. Arrieta, J. L. Pizarro, M. I. Arriortua and F. P. Cossío, Org. Biomol. Chem., 2008, 6, 1763 DOI: 10.1039/B719641E
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
