High stereoselectivity in chelation-controlled intermolecular Heck reactions with aryl chlorides, vinyl chlorides and vinyl triflates†
Abstract
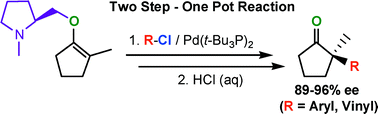
Highly stereoselective chelation-controlled Pd(0)-catalyzed β-arylations and β-vinylations of a five-membered chiral,

 Please wait while we load your content...
Please wait while we load your content...