Controlling cis/trans-selectivity in intramolecular Diels–Alder reactions of benzo-tethered, ester linked 1,3,9-decatrienes†
Abstract
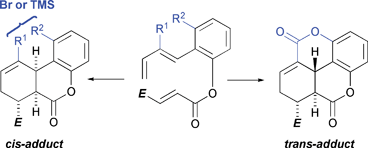
Predictions from DFT (B3LYP/6-31 G(d))-computed stereoisomer product distributions for intramolecular Diels–Alder (IMDA) reactions have been successfully replicated in the laboratory. Benzo-tethered hexadienyl acrylates generally undergo moderately trans-selective IMDA reactions which, as suggested by DFT calculation, arise from two opposing transition structure (TS) features: stabilising secondary orbital interactions, which are stronger in the cis-TSs, and stabilising π-conjugative interactions between the benzo moiety and the 1,3-diene component – which are stronger in trans-TSs. Substrates carrying a removable substituent (i.e. Br or TMS) at C3 of the

 Please wait while we load your content...
Please wait while we load your content...