Efficient synthesis of (+)-1,8,8a-tri-epi-swainsonine, (+)-1,2-di-epi-lentiginosine, (+)-9a-epi-homocastanospermine and (−)-9-deoxy-9a-epi-homocastanospermine from a d-glucose-derived aziridine carboxylate, and study of their glycosidase inhibitory activities†
Abstract
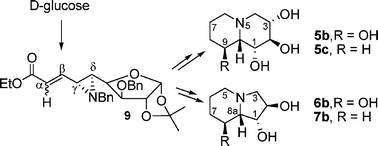
The utility of a D-glucose-derived aziridine carboxylate was demonstrated for the synthesis of polyhydroxylated quinolizidine and indolizidine

 Please wait while we load your content...
Please wait while we load your content...