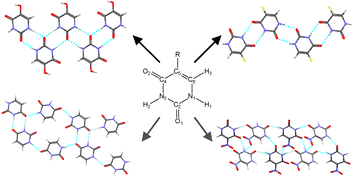
A search of the Cambridge Structural Database for crystal structures of 5-substituted uracils shows that, although there is a recurrent motif with symmetric hydrogen bonding and interdigitation of the 5-substituent R, a range of other hydrogen bonded ribbons, sheets and three-dimensional motifs are possible. In order to try and rationalize this, we have performed a combination of experimental studies and computational searches for low energy structures for the 12 simple 5-substituted uracils with R = H, CH3, CH2CH3, CHCH2, CN, OH, NH2, NO2, F, Cl, Br and I. Crystallization experiments on these compounds yielded the first single crystal X-ray determinations of 5-ethyluracil and 5-cyanouracil, as well as low temperature redeterminations of the disordered structures of 5-chlorouracil and 5-bromouracil. The lattice energies were calculated for the known crystal structures and compared with the computed lattice energy landscape for each molecule (except R = Br and I). Although the symmetric ribbon motif often dominates the computed crystal energy landscape, all of the molecules show a variety of different hydrogen bonding structures within a small energy range (5 kJ mol−1) of the global minimum and exhibit quite a diverse range of energetically competitive motifs. Thus, the range of crystallization outcomes, from polymorphism and other multiple forms, to the difficulty in growing single crystals (R = CHCH2 and NH2) probably reflects the sensitivity of the various hydrogen bonding motifs to the substituent and limited range of crystallization conditions that can be applied.

 Please wait while we load your content...
Please wait while we load your content...