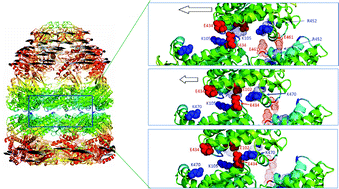
Despite significant efforts toward understanding the molecular basis of allosteric communication, the mechanisms by which local energetic and conformational changes cooperatively diffuse from ligand-binding sites to distal regions across the 3-dimensional structure of allosteric proteins remain to be established. Recent experimental and theoretical evidence supports the view that allosteric communication is facilitated by the intrinsic ability of the biomolecules to undergo collective changes in structure, triggered by ligand binding. Two groups of studies recently proved to provide insights into such intrinsic, structure-induced effects: elastic network models that permit us to visualize the cooperative changes in conformation that are most readily accessible near native state conditions, and information-theoretic approaches that elucidate the most efficient pathways of signal transmission favored by the overall architecture. Using a combination of these two approaches, we highlight, by way of application to the bacterial chaperonin complex GroEL-GroES, how the most cooperative modes of motion play a role in mediating the propagation of allosteric signals. A functional coupling between the global dynamics sampled under equilibrium conditions and the signal transduction pathways inherently favored by network topology appears to control allosteric effects.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...