Hydrogen-bond stabilized columnar discotic benzenetrisamides with pendant triphenylene groups†
Abstract
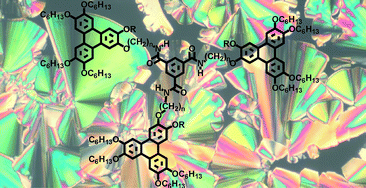
A series of 1,3,5-benzenetrisamide derivatives with three hexaalkoxytriphenylene pendant groups were prepared, in which the triphenylene groups are connected to the central 1,3,5-benzenetrisamide core through a flexible spacer. The length of this spacer, as well as the size of the ortho-substituent at the triphenylene core, influences the columnar packing of these molecules. All compounds show liquid crystalline behavior with high isotropization temperatures. Different columnar hexagonal phases (Colh, Colhp, Colobl and Colr) have been identified using

 Please wait while we load your content...
Please wait while we load your content...