Synthesis and through-bond spin interaction of stable 1,3-phenylene linked poly(phenothiazine cation radical)†
Abstract
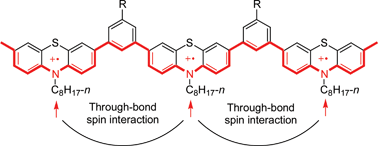
A 1,3-phenylene linked poly(phenothiazine cation radical) was synthesized and its through-bond spin interaction was investigated. Monomer and dimer models were also synthesized to investigate the behaviour of spins in poly(phenothiazine cation radical). X-Ray crystallographic analysis of the monomer model showed that it has a near-planar structure, which is favourable for spin interaction through π-conjugation. MO calculation of the dimer model showed its exchange integral J (+31.4 K) is larger by about two times than that of diaminoxyl (+14.5 K) which is a dimer model of a polyradical studied previously. The spin state of poly(phenothiazine cation radical) was S = 2/2–3/2 in the ground state, indicating that, taking into account the average degree of polymerization (5–6) and the spin concentration (0.77 spins per repeating unit), 2–3 spins out of 4–5 spins per molecule were ferromagnetically aligned.

 Please wait while we load your content...
Please wait while we load your content...