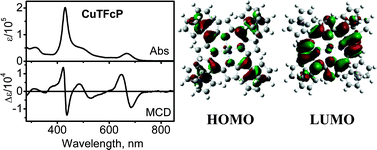
H2TFcP [TFcP = 5,10,15,20-tetraferrocenyl porphyrin(2−)] was prepared by a direct tetramerization reaction between pyrrole and ferrocene carbaldehyde in the presence of a BF3catalyst, while the series of MTFcP (M = Zn, Ni, Co and Cu) were prepared by a metallation reaction between H2TFcP and respective metal acetates. All compounds were characterized by UV-vis and MCD spectroscopy, APCI MS and MS/MS methods, high-resolution ESI MS and XPS spectroscopy. Diamagnetic compounds were additionally characterized using 1H and 13C NMR methods, while the presence of low-spin iron(II) centers in the neutral compounds was confirmed by Mössbauer spectroscopy and by analysis of the XPS Fe 2p peaks, revealing equivalent Fe sites. XPS additionally showed the influence on Fe 2p binding energies exerted by the distinct central metal ions. The conformational flexibility of ferrocene substituents in H2TFcP and MTFcP, was confirmed using variable-temperature NMR and computational methods. Density functional theory predicts that α,β,α,β atropisomers with ruffled porphyrin cores represent minima on the potential energy surfaces of both H2TFcP and MTFcP. The degree of non-planarity is central-metal dependent and follows the trend: ZnTFcP < H2TFcP ∼ CuTFcP < CoTFcP < NiTFcP. In all cases, a set of occupied, predominantly ferrocene-based molecular orbitals were found between the highest occupied and the lowest unoccupied, predominantly porphyrin-based molecular orbitals. The vertical excitation energies of H2TFcP were calculated at the TDDFT level and confirm the presence of numerous predominantly metal-to-ligand charge-transfer bands coupled via configurational interaction with expected intra-ligand π–π* transitions.

 Please wait while we load your content...
Please wait while we load your content...