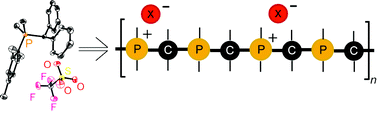
The chemical functionality of poly(methylenephosphine) n-Bu[MesP–CPh2]nH (2) is examined in reactions with two isoelectronic species, namely BH3 and CH3+. The potential reactivity of polymer 2 is modelled by examining the reactivity of molecular phosphines bearing similar substituents as the polymer. In particular, the phosphine–borane adducts Mes(Me)P(BH3)–CPh2H (4a) and Mes(Me)P(BH3)–CPh2SiMe2H (4b) are prepared from the reaction of BH3·SMe2 with Mes(Me)P–CPh2H (3a) or Mes(Me)P–CPh2SiMe2H (3b), respectively. Treating 3a with MeOTf affords the methylated model compound, [Mes(Me)2P–CPh2H]OTf (5). X-Ray crystal structures are reported for each model compound. The reaction of n-Bu[MesP–CPh2]nH (Mn = 3.89 × 104, PDI = 1.34) with BH3·SMe2 affords the phosphine–borane polymern-Bu[MesP(BH3)–CPh2]nH (6) (Mn = 4.13 × 104, PDI = 1.26). In contrast, methylation of phosphine polymer 2 gives n-Bu[MesP–CPh2]x–/–[MesP(Me)–CPh2]yH·(OTf)y (7) where approximately 50% of the phosphine moieties are methylated (from 31P NMR).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...