The β-keto phosphorus ylides (n-Bu)3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 6, (t-Bu)2PhP
CHC(O)Ph 6, (t-Bu)2PhP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 7, (t-Bu)Ph2P
CHC(O)Ph 7, (t-Bu)Ph2P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 8, (n-Bu)2PhP
CHC(O)Ph 8, (n-Bu)2PhP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 9, (n-Bu)Ph2P
CHC(O)Ph 9, (n-Bu)Ph2P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 10, Me2PhP
CHC(O)Ph 10, Me2PhP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 11 and Ph3P
CHC(O)Ph 11 and Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)(o-OMe-C6H4) 12 have been synthesized in 80–96% yields. The Ni(II) complexes [N
CHC(O)(o-OMe-C6H4) 12 have been synthesized in 80–96% yields. The Ni(II) complexes [N![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph2PCH
iPh{Ph2PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )(o-OMeC6H4)}(PPh3)] 13, [N
)(o-OMeC6H4)}(PPh3)] 13, [N![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph(t-Bu)PCH
iPh{Ph(t-Bu)PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(PPh3)] 15, [N
)Ph}(PPh3)] 15, [N![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{(n-Bu)2PCH
iPh{(n-Bu)2PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(PPh3)] 16 and [N
)Ph}(PPh3)] 16 and [N![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph(n-Bu)PCH
iPh{Ph(n-Bu)PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(PPh3)] 17 have been prepared by reaction of equimolar amounts of [Ni(COD)2] and PPh3 with the β-keto phosphorus ylides 12 or 8–10, respectively, and characterized by 1H and 31P{1H} NMR spectroscopy. NMR studies and the crystal structure determination of 13 indicated an interaction between the hydrogen atom of the C–H group α to phosphorus and the ether function. The complexes [N
)Ph}(PPh3)] 17 have been prepared by reaction of equimolar amounts of [Ni(COD)2] and PPh3 with the β-keto phosphorus ylides 12 or 8–10, respectively, and characterized by 1H and 31P{1H} NMR spectroscopy. NMR studies and the crystal structure determination of 13 indicated an interaction between the hydrogen atom of the C–H group α to phosphorus and the ether function. The complexes [N![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph2PCH
iPh{Ph2PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(Py)] 18, [N
)Ph}(Py)] 18, [N![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph(t-Bu)PCH
iPh{Ph(t-Bu)PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(Py)] 19, [N
)Ph}(Py)] 19, [N![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{(n-Bu)2PCH
iPh{(n-Bu)2PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(Py)] 20, [N
)Ph}(Py)] 20, [N![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph(n-Bu)PCH
iPh{Ph(n-Bu)PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(Py)] 21 and [N
)Ph}(Py)] 21 and [N![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Me2PCH
iPh{Me2PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(Py)] 22 have been isolated from the reactions of [Ni(COD)2] and an excess of pyridine with the β-keto phosphorus ylides Ph3PCH
)Ph}(Py)] 22 have been isolated from the reactions of [Ni(COD)2] and an excess of pyridine with the β-keto phosphorus ylides Ph3PCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(O)Ph 3 or 8–11, respectively, and characterized by 1H and 31P{1H} NMR spectroscopy. Ligands 3, 8, 10 and 12 have been used to prepare in situ oligomerization catalysts by reaction with one equiv. of [Ni(COD)2] and PPh3 under an ethylene pressure of 30 or 60 bar. The catalyst prepared in situ from 12, [Ni(COD)2] and PPh3 was the most active of the series with a TON of 12 700 mol C2H4 (mol Ni)−1 under 30 bar ethylene. When the β-keto phosphorus ylide 8 was reacted in situ with three equiv. of [Ni(COD)2] and one equiv. of PPh3 under 30 bar of ethylene, ethylene polymerization was observed with a TON of 5500 mol C2H4 (mol Ni)−1.
C(O)Ph 3 or 8–11, respectively, and characterized by 1H and 31P{1H} NMR spectroscopy. Ligands 3, 8, 10 and 12 have been used to prepare in situ oligomerization catalysts by reaction with one equiv. of [Ni(COD)2] and PPh3 under an ethylene pressure of 30 or 60 bar. The catalyst prepared in situ from 12, [Ni(COD)2] and PPh3 was the most active of the series with a TON of 12 700 mol C2H4 (mol Ni)−1 under 30 bar ethylene. When the β-keto phosphorus ylide 8 was reacted in situ with three equiv. of [Ni(COD)2] and one equiv. of PPh3 under 30 bar of ethylene, ethylene polymerization was observed with a TON of 5500 mol C2H4 (mol Ni)−1.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 6, (t-Bu)2PhP
CHC(O)Ph 6, (t-Bu)2PhP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 7, (t-Bu)Ph2P
CHC(O)Ph 7, (t-Bu)Ph2P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 8, (n-Bu)2PhP
CHC(O)Ph 8, (n-Bu)2PhP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 9, (n-Bu)Ph2P
CHC(O)Ph 9, (n-Bu)Ph2P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 10, Me2PhP
CHC(O)Ph 10, Me2PhP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)Ph 11 and Ph3P
CHC(O)Ph 11 and Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC(O)(o-OMe-C6H4) 12 have been synthesized in 80–96% yields. The
CHC(O)(o-OMe-C6H4) 12 have been synthesized in 80–96% yields. The ![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph2PCH
iPh{Ph2PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )(o-OMeC6H4)}(PPh3)] 13, [
)(o-OMeC6H4)}(PPh3)] 13, [![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph(t-Bu)PCH
iPh{Ph(t-Bu)PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(PPh3)
)Ph}(PPh3)![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{(n-Bu)2PCH
iPh{(n-Bu)2PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(PPh3)
)Ph}(PPh3)![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph(n-Bu)PCH
iPh{Ph(n-Bu)PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(PPh3)]
)Ph}(PPh3)]![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph2PCH
iPh{Ph2PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(Py)
)Ph}(Py)![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph(t-Bu)PCH
iPh{Ph(t-Bu)PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(Py)
)Ph}(Py)![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{(n-Bu)2PCH
iPh{(n-Bu)2PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(Py)
)Ph}(Py)![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Ph(n-Bu)PCH
iPh{Ph(n-Bu)PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(Py)
)Ph}(Py)![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) iPh{Me2PCH
iPh{Me2PCH![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) C(
C(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O
O![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )Ph}(Py)]
)Ph}(Py)]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(O)Ph 3 or 8–11, respectively, and characterized by 1H and
C(O)Ph 3 or 8–11, respectively, and characterized by 1H and 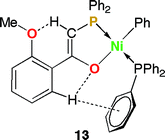

 Please wait while we load your content...
Please wait while we load your content...