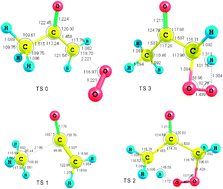
CBS-QB3 calculations have been used to determine thermochemical and kinetic parameters of the isomerisation and decomposition reactions of the acetonylperoxy radical, CH3C(O)CH2OO˙, which has been formed via the reaction of acetonyl radical with O2 leading to the formation of an energised peroxy adduct with a calculated well depth of near 111 kJ mol−1. This species can undergo subsequent 1,5 and 1,3 H-shifts to give the primary and secondary radicals: C˙H2C(O)CH2OOH and CH3C(O)C˙HOOH, respectively, or rearrange to give a 3-methyl-1,2-dioxetan-3-yloxy radical. Rate constants for isomerisation and subsequent decomposition have been estimated using canonical variational transition state theory with small curvature tunneling CVT/SCT. The variational effect for the isomerisation channels is only moderate but the tunneling correction is significant at temperatures up to 1000 K; the formation of a primary radical by a 1,5-shift is the main reaction channel and the competition with the secondary one starts only at around 1500 K. The fate of the primary acetonylhydroperoxy radical is predominantly to form oxetan-3-one while the ketene and 1-oxy-3-hydroxyacetonyl radical channels only compete with the formation of oxetan-3-one at temperatures >1200 K. In addition, consistent and reliable enthalpies of formation have been computed for the molecules acetonylhydroperoxide, 1,3-dihydroxyacetone, methylglyoxal and cyclobutanone, and for some related radicals.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...