Aqueous peptides as experimental models for hydration water dynamics near protein surfaces
Abstract
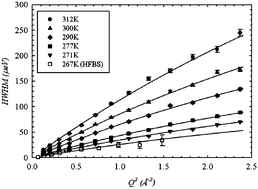
We report quasi-elastic neutron scattering experiments to contrast the water dynamics as a function of temperature for hydrophilic and amphiphilic
- This article is part of the themed collections: Water at interfaces and Highlighting Canadian research in PCCP
 Please wait while we load your content...
Please wait while we load your content...