New mechanistic insight into electronically excited CO–NiO(100): a quantum dynamical analysis†
Abstract
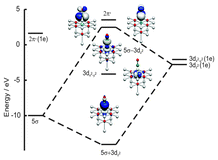
In a recent study Redlich et al. [Redlich et al., Chem. Phys. Lett. 2006, 420, 110] measured the velocity distribution of CO molecules desorbing from a NiO(100) surface after irradiation with an ultraviolet (UV) laser pulse. Due to the complexity of the involved processes no experimental evidence on the excitation and desorption mechanism could be obtained. In recent ab initio studies Mehdaoui et al. [Mehdaoui et al., Phys. Rev. Lett. 2007, 98, 037601] have shown that a 5σ → 2π* (a 3Π) like transition within the CO adsorbate is most likely the crucial excitation step in the CO–NiO(100) system. At first sight this seems unlikely, since the interaction of CO molecules with the NiO(100) surface is very weak (−0.30 eV) and the corresponding CO gas phase transition energy is about 1.5 eV higher than the laser pulse energy of 4.66 eV used in the experiment. In this work we give further insight into relevant electronically excited states and identify the desorption mechanism by analysing the dynamical processes after laser excitation by quantum dynamical wave packet simulations on the basis of three-dimensional (3D) ab initio potential energy surfaces. The results corroborate the so far discussed excitation mechanism, which proposes the formation of a genuine C–Ni bond as the driving force for photodesorption, as the crucial excitation step.

 Please wait while we load your content...
Please wait while we load your content...