A combined theoretical–experimental study on the acidity of WOx-ZrO2 systems
Abstract
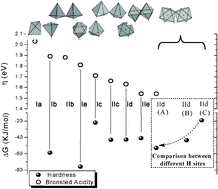
This work provides a chemical approach to the relationship between structure and electronic behavior of the active surface of the WOx-ZrO2 system as a function of W loads. This study shows that the electronic hardness (η), the Lewis and Brønsted acidity are functions of the local coordination and of the polymerization degree of the WOx domain. From theoretical calculations the observed behavior in the WOx-ZrO2 system is explained: the Brønsted acidity increases while the Lewis acidity decreases as the W centers go from tetrahedral to octahedral coordination and as the condensation degree of the WOx domain increases. Our results also indicate that not all the Brønsted sites in the WOx domains are equally acid, and that as the W load increases the most acid sites decrease in number due to the condensation process. This finding also means a decrease on the average acidity per H site. Additionally, our results suggest that for surface densities in the 4–7 W nm−2 range, mainly dimeric-tungstate species are present. A maximum in Brønsted acidity was observed for a W surface density about 7 W nm−2.

 Please wait while we load your content...
Please wait while we load your content...