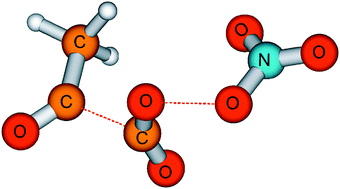
Reactions of methylglyoxyl and methylglyoxylperoxy radicals were investigated at a total pressure of 1 bar in oxygen. Methylglyoxyl radicals were generated by stationary photolysis of Br2–CH3C(O)C(O)H–NO2–O2–N2 mixtures at wavelengths ≥480 nm and of Cl2–CH3C(O)C(O)H–NO2–O2–N2 mixtures in the wavelength range 315–460 nm. In the bromine system, rate constant ratios for the reactions CH3C(O)CO → CH3CO + CO (kdis) and CH3C(O)CO + O2 → CH3C(O)C(O)O2 (kO2) were measured as a function of temperature in the range 275–311 K. Assuming the constant value kO2 = 5.1 × 10−12 cm3 molecule−1 s−1 for our reaction conditions, kdis = 1.2 × 1010.0±0.7 × exp(−11.7 ± 3.8 kJ mol−1/RT) s−1 (2σ errors) was obtained for ptot = 1 bar (M = O2), in good agreement with the kinetic parameters calculated by Méreau et al. [R. Méreau, M.-T. Rayez, J.-C. Rayez, F. Caralp and R. Lesclaux, Phys. Chem. Chem. Phys., 2001, 3, 4712]. CH3C(O)C(O)O2 radicals oxidise NO2, forming NO3, CH3CO and CO2. This experimental result is supported by DFT and ab initio calculations. Possible mechanisms for the observed formation of several % of ketene and bromoacetyl peroxynitrate are discussed. Use of Cl rather than Br atoms to abstract the aldehydic H atom from methylglyoxal leads to chemically activated CH3C(O)CO radicals, thus substantially increasing the fraction of CH3C(O)CO radicals that decompose rather than add O2.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...