Designed molecular propellers based on tetraarylterephthalamide and their chiroptical properties induced by biased helicity through transmission of point chirality†
Abstract
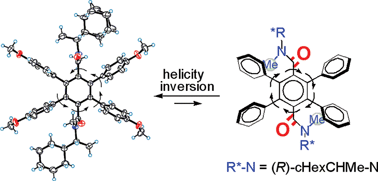
The syn-atropisomers of the title bis(tertiary amide)s were designed as six-bladed molecular propellers based on the “directing effects” of

 Please wait while we load your content...
Please wait while we load your content...