Anti-Markovnikov hydroarylation of styrenes catalyzed by an in situ generated ruthenium complex†
Abstract
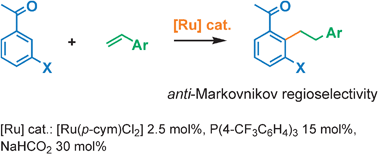
An efficient and practical catalytic system for the anti-Markovnikov ruthenium-catalyzed hydroarylation of styrenes with acetophenone, allowing a straightforward access to bibenzyl backbones, is described for the first time: this process, involving regioselective C–H bond activation, is complementary to a Friedel–Crafts type reaction giving the branched adduct.

 Please wait while we load your content...
Please wait while we load your content...