Photostability of pentacene and 6,13-disubstituted pentacene derivatives: a theoretical and experimental mechanistic study
Abstract
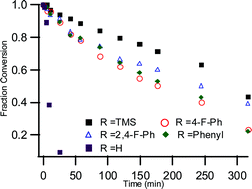
Computational and experimental studies have been performed to investigate the photostability of a series of 6,13-bis(arylalkynyl)-substituted
- This article is part of the themed collection: Photodynamic therapy and photodetection

 Please wait while we load your content...
Please wait while we load your content...