Synthesis of imidazolidin-2-one-4-carboxylate and of (tetrahydro)pyrimidin-2-one-5-carboxylatevia an efficient modification of the Hofmann rearrangement†
Abstract
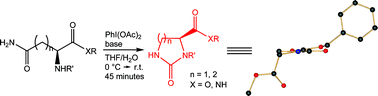
A mild and efficient methodology for the rearrangement of protected asparagine and protected glutamine is reported; good results are obtained with a wide selection of protecting groups.

 Please wait while we load your content...
Please wait while we load your content...