Enantioselective addition of nitromethane to α-keto esters catalyzed by copper(ii)–iminopyridine complexes
Abstract
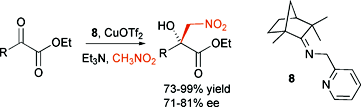
The copper complex of a chiral iminopyridine easily prepared from (R)-(−)-fenchone and picolylamine catalyzes the enantioselective Henry (nitroaldol) reaction between

 Please wait while we load your content...
Please wait while we load your content...