Knipholone and related 4-phenylanthraquinones: structurally, pharmacologically, and biosynthetically remarkable natural products
Abstract
Covering: up to May 2008
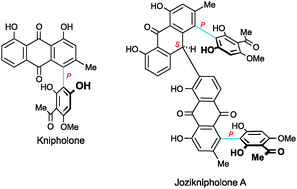
This review gives the first comprehensive overview of the rapidly emerging young class of 4-phenylanthraquinones isolated from the three genera Bulbine, Bulbinella, and Kniphofia, all from the plant family Aphodelaceae: their occurrence, structural elucidation (with particular emphasis on the phenomenon of axial chirality), partial and total synthesis, their pharmacological (i.a.,

 Please wait while we load your content...
Please wait while we load your content...