Covering: primarily 2003–2007
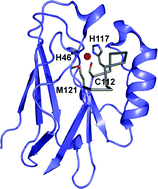
Many approaches are being used to engineer metalloproteins, with most of these informed by, and aiming to further elucidate, the basic structural requirements for biological metal centers. Cupredoxins are type 1 (T1) copper-containing electron transfer (ET) proteins with a β-barrel fold that is thought to constrain metal site structure. The T1 copper ion is bound by ligands mainly originating from a single active site loop whose length and structure varies. This Highlight article will focus on protein engineering studies which have investigated the role of the metal-binding loop for active site integrity and functionality. Scaffold differences are present within the cupredoxin family and their influence has also been assessed. Given the widespread occurrence of β-barrel domains in nature, and the array of metal sites in proteins composed of loop regions, the studies described on this model system have implications for a variety of metalloproteins.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...