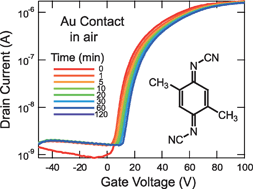
N-channel organic field-effect transistors with stable performance at ambient conditions are fabricated on the basis of an electron-accepting molecule, dimethyldicyanoquinone diimine (DMDCNQI). The transistors are investigated by varying source and drain electrode materials: Au, Ag, Cu, and a highly conducting organic charge-transfer salt, (tetrathiafulvalene)(tetracyanoquinodimethane) [(TTF)(TCNQ)]. The devices with the Au electrode show lowest contact resistance and highest electron mobility (0.011 cm2V−1 s−1 for bottom-contact configuration), and the performance decreases in the order of Au > (TTF)(TCNQ) > Ag > Cu. This order does not seem related to the metal work functions, but is attributed to the organic–metal interfacial potentials. DMDCNQI forms highly conducting charge-transfer complexes with Ag and Cu, but the complex layer increases the interfacial potential as well as the electron-injection barrier and also increases the off-current for short channel devices. The air stability is not determined solely by the organic semiconductor but is considerably influenced by the electrode materials.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...