A comparative study of the electronic structures of SrCu2O2 and PbCu2O2 by density functional theory, high resolution X-ray photoemission and electron paramagnetic resonance spectroscopy†
Abstract
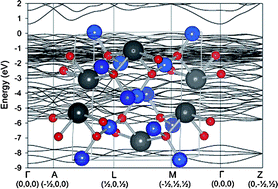
The electronic structures of SrCu2O2 and PbCu2O2 have been studied by density functional theory calculations in conjunction with

 Please wait while we load your content...
Please wait while we load your content...