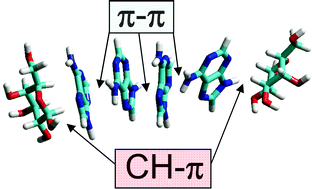
CH–Pi stacking interactions between carbohydrates and aromatic compounds play a central role in biomolecular recognition, especially in lectin–sugar and protein–glycolipid systems. In the present study, we have measured the solubility of the sparingly soluble aromatic base adenine in presence of various saccharides as an approach to investigate the interaction between adenine and sugars. Above 82.5 mM, adenine solutions gradually formed a crystalline precipitate which could be quantified by spectrophotometric turbidity measurements. Precipitation of adenine was increased by salts (NaCl and NaF) whereas it was prevented by DMSO, in agreement with the involvement of hydrophobic interactions (π–π stacking) in the vertical stacking of adenine molecules. Several monosaccharides and disaccharides were found to increase adenine solubility, with the following order: D-galactose = D-lactose > D-sucrose > D-glucose = D-maltose > D-ribose > D-fructose. Molecular mechanics simulations indicated that the potent cosolvent effect of β-D-galactopyranose was probably mediated by CH–π stacking interactions between its apolar surface and the aromatic structure of adenine. The polar OH groups of the sugars interacted with surrounding water molecules, ensuring the solubility of sugar–adenine complexes. In contrast, β-D-fructofuranose, which has two polar faces, did not stack onto adenine and had a weak cosolvent effect. CH–π stacking interactions were also demonstrated between 6-methylpurine and the sugar head group of glycolipids (glucosyl-, galactosyl- and lactosylceramide) but not with the charged head group of phosphatidylinositol-4,5-diphosphate. These data indicate that galactose-containing molecules have a high stacking propensity for aromatic compounds such as adenine, due to the specific structure of the galactose cycle.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...